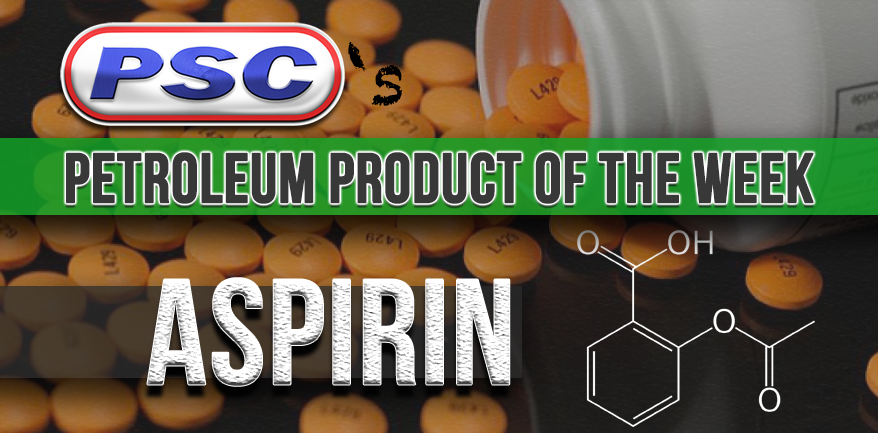
Petroleum Product of the Week: Aspirin
By on Nov 18 2016
It seems pretty safe to say that November has been a month of headaches for a lot of people.
And if avoiding social media and other news outlets haven't been much help, you've probably (at some point) reached for some over-the-counter pain relief.
Maybe some aspirin?
Aspirin is a popular medication used to treat pain, fever, inflammation, and is even sometimes used to treat or prevent heart attacks, strokes, and chest pain.
History of Aspirin

Believe it or not, aspirin (AKA acetylsalicylic acid or ASA) is an antique medical substance.
Ancient Egyptians used medicines made from willow and other salicylate-rich plants from the second millennium BC. Hippocrates (an Ancient Greek physician, considered the father of modern medicine) notes the use of salicylic tea to reduce fevers circa 400 BC. In 1763, a priest of the Church of England is credited with having isolated the active ingredient on aspirin in his discovery of salicylic acid.
It was French chemist Charles Frédéric Gerhardt who, in 1853, treated acetyl chloride with sodium salicylate to produce acetylsalicylic acid (aspirin as we know it) for the first time. During the latter half of the 19th century, other academic chemists worked to establish the compound's chemical structure and came up with better methods of synthesis.
In 1897, scientists at Bayer (a drug and dye firm) began investigating ASA as a replacement for the standard (more common and more irritating) salicylate meds. Within 2 years, Bayer named their drug "Aspirin." They sold it worldwide.
Technically, Aspirin was trademarked by Bayer and was a brand name, rather than a generic drug. However, Bayer lost (or sold) their trademark in many countries. Due to the drug's popularity during the first half of the 20th century, companies competed among each other for aspirin brands and products.
Aspirin's popularity declined with the development of acetaminophen in 1956 and ibuprofen in 1962. Aspirin sales revived once it was discovered that the drug was an effective anti-clotting agent that can reduce the risk of clotting diseases. Sales grew, and remain relatively strong today--especially for use as a preventative treatment for heart attacks and strokes.
Where's the Petroleum?
While early aspirin-like medicines were made from natural sources like willow bark, modern aspirin is completely chemically synthesized. This is where the petroleum comes in.
You might be used to taking oil supplements like fish oil, krill oil, or even flaxseed oil. But the "oil" in aspirin isn't like that. It's not even oil at all. Aspirin simply contains a chemical that just so happens to be a natural part of crude oil and gasoline (it's also formed by volcanoes and forest fires).
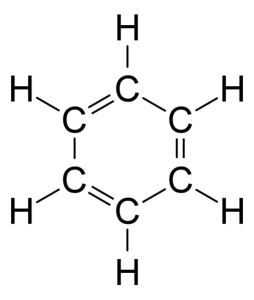
The chemical in question is benzene.
Benzene is one of the most widely used chemicals in the US today. It is used predominately as a starting material in making other chemicals--including aspirin and other drugs.
One way benzene is obtained is by distillation-refining petroleum. Once the Benzene is produced, it is reacted sequentially with sulphuric acid and sodium hydroxide to give sodium phenolate. it undergoes a chemical reaction (the Kolbe-Schmidt reaction, if you want specifics) to give sodium salicylate, which is then acidified to give salicylic acid. The salicylic acid is then reacted with acetic anhydride in the presence of an acid catalyst, usually sulphuric or phosphoric acid, to give acetyl salicylic acid. Or, in other words, aspirin. Phew.
Safety tip: as aspirin ages, it has the potential to decompose and return to salicyclic acid and acetic acid. So if you uncover an old bottle of aspirin and it smells anything like vinegar, it's probably not safe to use. Go pick yourself up another bottle to relieve your post-election headaches.
Sources:
https://en.wikipedia.org/wiki/Aspirin
https://en.wikipedia.org/wiki/History_of_aspirin
http://www.cancer.org/cancer/cancercauses/othercarcinogens/intheworkplace/benzene
https://chempics.wordpress.com/2013/09/18/acetyl-salicylic-acid-aspirin/